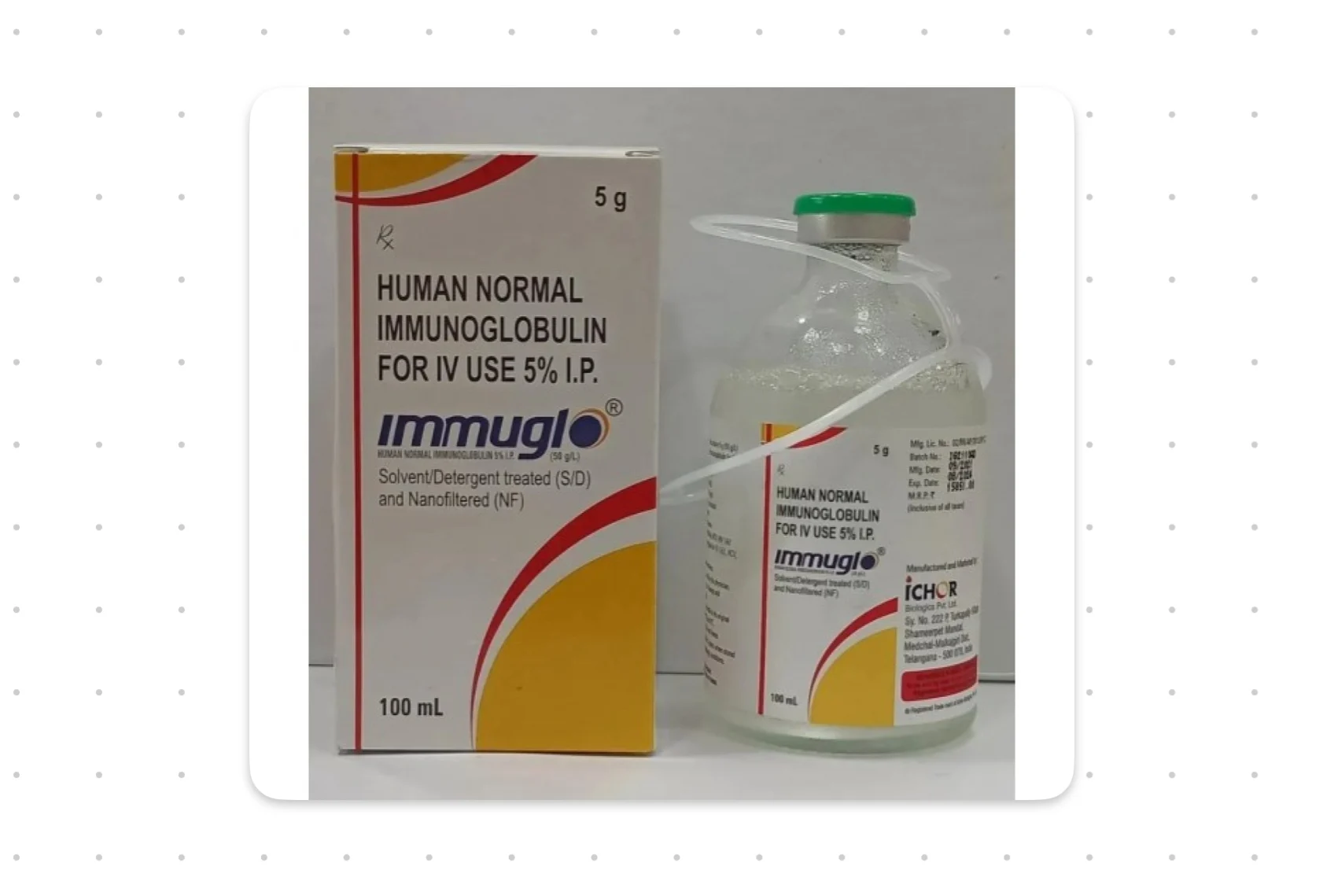
The Maldives Food and Drug Authority (MFDA) has issued a warning to consumers regarding quality concerns associated with a batch of human normal immunoglobulin IV manufactured in India.
The MFDA reported that four batches of Normal Immunoglobulin for Intravenous Use BP 5 percent, produced by Ico Biologics Ltd., have been flagged due to quality issues. This matter has also been communicated to the National Medicine Regulatory Authority and the World Health Organization for further investigation.
In its statement, the MFDA clarified that the affected IV preparations are not on the list of drugs approved for import and sale in the Maldives. Importantly, the agency confirmed that no batches of this IV have been imported into the country.
Despite the lack of imports, the MFDA urges the public to remain vigilant, particularly as India and the Maldives are common destinations for medical treatment. As a precautionary measure, the MFDA will enhance its inspection of medicines imported into the Maldives to ensure the safety and quality of health products available to consumers.
The MFDA reported that four batches of Normal Immunoglobulin for Intravenous Use BP 5 percent, produced by Ico Biologics Ltd., have been flagged due to quality issues. This matter has also been communicated to the National Medicine Regulatory Authority and the World Health Organization for further investigation.
In its statement, the MFDA clarified that the affected IV preparations are not on the list of drugs approved for import and sale in the Maldives. Importantly, the agency confirmed that no batches of this IV have been imported into the country.
Despite the lack of imports, the MFDA urges the public to remain vigilant, particularly as India and the Maldives are common destinations for medical treatment. As a precautionary measure, the MFDA will enhance its inspection of medicines imported into the Maldives to ensure the safety and quality of health products available to consumers.