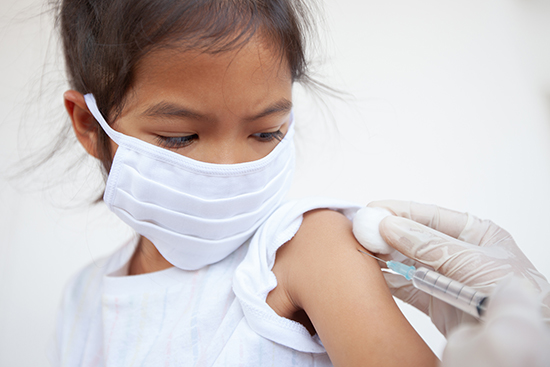
As per sources familiar with the situation, top U.S. health officials believe that Pfizer’s Covid-19 vaccine could be authorized for children aged 5-11 years old by the end of October.
The decision on whether to authorize a vaccine for younger children is eagerly anticipated by millions of Americans, particularly parents whose children started school in recent weeks amid a wave of infections driven by the Delta variant.
Top US infectious disease expert Dr. Anthony Fauci outlined the timetable during an online town hall meeting attended by thousands of staff at the National Institutes of Health (NIH) on Friday, according to one of the sources.
If Pfizer submits its emergency use authorization (EUA) by the end of September, and the data support its use, “by the time we get to October, the first couple of weeks of October... the Pfizer product will likely be ready,” Fauci said.
According to one of the sources, Fauci also said that Moderna Inc. will likely take about three weeks longer than Pfizer to collect and analyze its data on children aged 5-11, according to the source. He estimated that a decision on the Moderna shot could come around November.
Pfizer has previously said that it would have data on children aged 5-11 ready in September and planned to submit for a EUA shortly after. Previously, federal health regulators, including Fauci, have suggested that an FDA decision might come in November or later. Moderna on Thursday told investors it expected data from its children’s study by the end of the year.
The decision on whether to authorize a vaccine for younger children is eagerly anticipated by millions of Americans, particularly parents whose children started school in recent weeks amid a wave of infections driven by the Delta variant.
Top US infectious disease expert Dr. Anthony Fauci outlined the timetable during an online town hall meeting attended by thousands of staff at the National Institutes of Health (NIH) on Friday, according to one of the sources.
If Pfizer submits its emergency use authorization (EUA) by the end of September, and the data support its use, “by the time we get to October, the first couple of weeks of October... the Pfizer product will likely be ready,” Fauci said.
According to one of the sources, Fauci also said that Moderna Inc. will likely take about three weeks longer than Pfizer to collect and analyze its data on children aged 5-11, according to the source. He estimated that a decision on the Moderna shot could come around November.
Pfizer has previously said that it would have data on children aged 5-11 ready in September and planned to submit for a EUA shortly after. Previously, federal health regulators, including Fauci, have suggested that an FDA decision might come in November or later. Moderna on Thursday told investors it expected data from its children’s study by the end of the year.