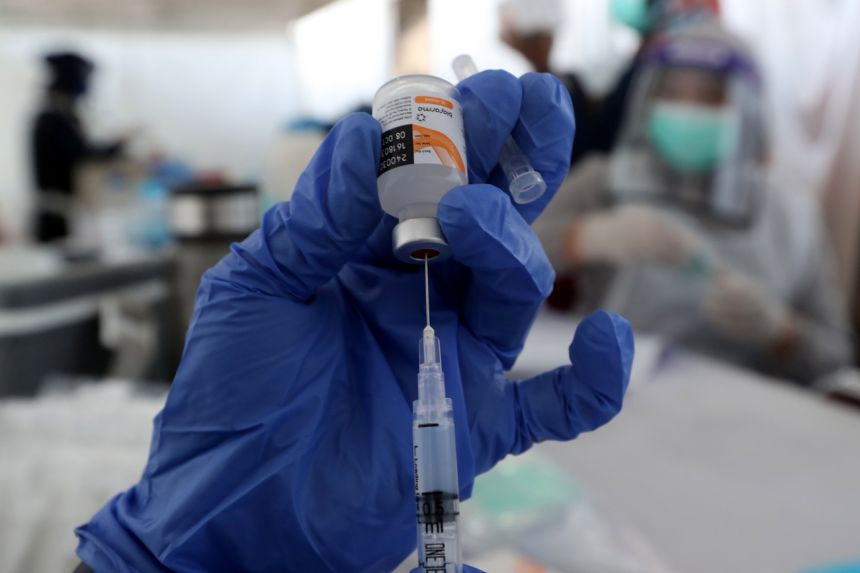
Indonesia's food and drug agency (BPOM) has recommended the coronavirus vaccine made by China's Sinovac Biotech for children aged 12 to 17, the country's Covid-19 task force said this Monday, as the country seeks to extend inoculations amid a surge in infections.
Indonesia has reported record daily rises in cases of more than 20,000 recently after the emergence of virus variants and travel after the Muslim fasting month has helped drive a new wave of infections.
Taskforce spokesman Wiku Adisasmito welcomed the food and drug agency's recommendation and said "the government invites the people to still wait for the issuance of emergency use approval from BPOM".
As per Taskforce data children aged 0-18 account for 12.6% of Indonesia's total Covid-19 infections.
With a new wave of infections, Indonesia is under pressure to speed up vaccinations, with hospitals in several designated red zones reporting overcapacity and 93 percent of isolation beds in Jakarta occupied as of Sunday.
Indonesia has been using Sinovac as the main plank for its vaccination program after receiving about 94 million doses, while it has received about 10 million made by AstraZeneca and Sinopharm. Under its vaccination program, Indonesia has inoculated about 13.18 million people, so far.
The World Health Organization (WHO) approved emergency use of Sinovac's vaccine this month, saying results showed it prevented symptomatic disease in 51% of recipients and prevented severe Covid-19 and hospital stays.
Indonesia has reported record daily rises in cases of more than 20,000 recently after the emergence of virus variants and travel after the Muslim fasting month has helped drive a new wave of infections.
Taskforce spokesman Wiku Adisasmito welcomed the food and drug agency's recommendation and said "the government invites the people to still wait for the issuance of emergency use approval from BPOM".
As per Taskforce data children aged 0-18 account for 12.6% of Indonesia's total Covid-19 infections.
With a new wave of infections, Indonesia is under pressure to speed up vaccinations, with hospitals in several designated red zones reporting overcapacity and 93 percent of isolation beds in Jakarta occupied as of Sunday.
Indonesia has been using Sinovac as the main plank for its vaccination program after receiving about 94 million doses, while it has received about 10 million made by AstraZeneca and Sinopharm. Under its vaccination program, Indonesia has inoculated about 13.18 million people, so far.
The World Health Organization (WHO) approved emergency use of Sinovac's vaccine this month, saying results showed it prevented symptomatic disease in 51% of recipients and prevented severe Covid-19 and hospital stays.