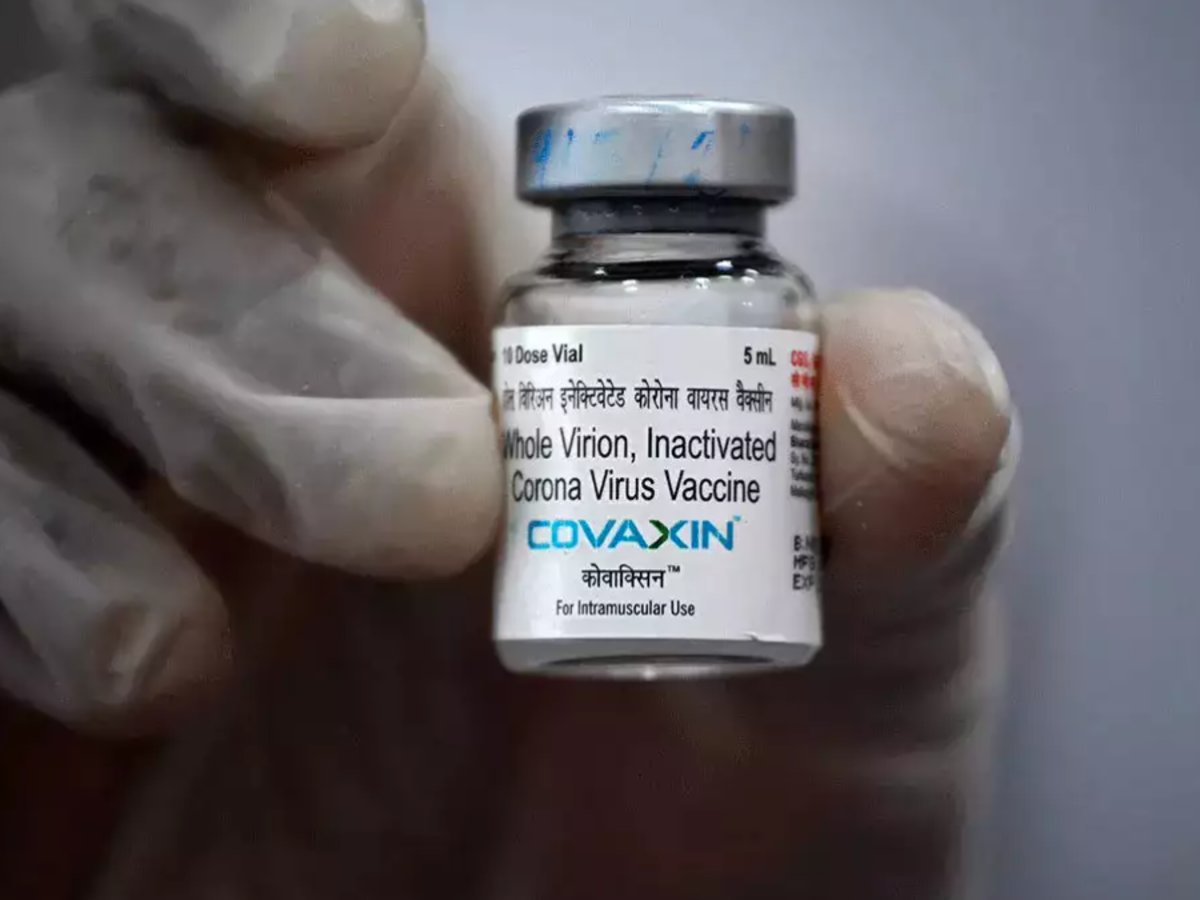
The World Health Organization (WHO) granted an emergency use license Wednesday to Covaxin, a coronavirus vaccine developed in India.
"The Technical Advisory Group, convened by WHO and made up of regulatory experts from around the world, has determined that the Covaxin vaccine meets WHO standards for protection against COVID-19, that the benefit of the vaccine far outweighs risks & the vaccine can be used," the organization said in a tweet.
The vaccine was also reviewed by WHO’s Strategic Advisory Group of Experts on Immunization (SAGE), and recommended use of this vaccine in two doses, with a dosing interval of four weeks, in all age groups 18 and above. WHO also said that the vaccine was recommended for use in two doses, with a dosing interval of four weeks, in all age groups 18 and above. However, the vaccine was not recommended to be used for the vaccination of pregnant women, as the available data on the vaccination of pregnant women with the vaccine are insufficient to assess vaccine safety or efficacy in pregnancy.
WHO said that Covaxin had 78% efficacy against Covid 19 of any severity, 14 or more days after the second dose, and is extremely suitable for low- and middle-income countries due to easy storage requirements.
Covaxin was developed by Bharat Biotech in partnership with the Indian Council of Medical Research, the government’s apex research body. The vaccine is made using a killed coronavirus to prompt an immune response and is given in two doses.
India’s drug regulator authorized Covaxin in January, months before extensive testing in people had been completed, prompting concern from health experts that the shot was given the nod prematurely. By mid-October, over 110 million jabs of the vaccine had been administered, making Covaxin the second-most used COVID-19 shot in India after AstraZeneca’s.
"The Technical Advisory Group, convened by WHO and made up of regulatory experts from around the world, has determined that the Covaxin vaccine meets WHO standards for protection against COVID-19, that the benefit of the vaccine far outweighs risks & the vaccine can be used," the organization said in a tweet.
The vaccine was also reviewed by WHO’s Strategic Advisory Group of Experts on Immunization (SAGE), and recommended use of this vaccine in two doses, with a dosing interval of four weeks, in all age groups 18 and above. WHO also said that the vaccine was recommended for use in two doses, with a dosing interval of four weeks, in all age groups 18 and above. However, the vaccine was not recommended to be used for the vaccination of pregnant women, as the available data on the vaccination of pregnant women with the vaccine are insufficient to assess vaccine safety or efficacy in pregnancy.
WHO said that Covaxin had 78% efficacy against Covid 19 of any severity, 14 or more days after the second dose, and is extremely suitable for low- and middle-income countries due to easy storage requirements.
Covaxin was developed by Bharat Biotech in partnership with the Indian Council of Medical Research, the government’s apex research body. The vaccine is made using a killed coronavirus to prompt an immune response and is given in two doses.
India’s drug regulator authorized Covaxin in January, months before extensive testing in people had been completed, prompting concern from health experts that the shot was given the nod prematurely. By mid-October, over 110 million jabs of the vaccine had been administered, making Covaxin the second-most used COVID-19 shot in India after AstraZeneca’s.