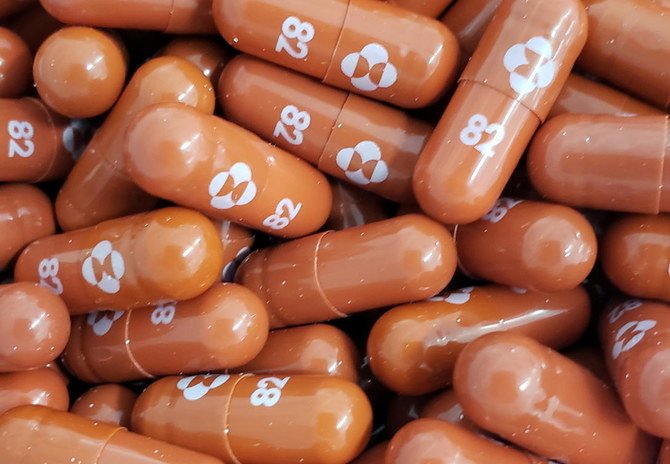
An antiviral pill developed by US drugmaker Merck & Co. could half the chances of dying or being hospitalized for those most at risk of contracting severe COVID-19, with experts hailing it as a potential breakthrough in how the virus is treated.
With success in reducing the most severe outcomes of the illness, clinical trials of the medication were stopped early so more people might benefit, drugmaker Merck & Co. announced Friday.
A five-day course of the drug, called molnupiravir, which is designed to introduce errors into the genetic code of the virus, was found to cut Covid hospitalizations and related deaths in half, according to a company press release. Merck said it intends to ask health officials in the United States and around the world for emergency authorization for the pill.
If it gets authorization, molnupiravir would be the first oral antiviral medication for COVID-19.
“This is going to change the dialogue around how to manage COVID-19,” Merck Chief Executive Robert Davis said.
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received molnupiravir within five days of Covid symptoms had about a 50 percent reduction in the rate of hospitalization and death as patients who received a placebo pill. The study tracked 775 adults with mild-to-moderate Covid who were considered at higher risk for severe disease due to health problems such as obesity, diabetes, or heart disease.
The results that were released by the company and have not been peer-reviewed. Merck said it plans to present them at a future medical meeting.
With success in reducing the most severe outcomes of the illness, clinical trials of the medication were stopped early so more people might benefit, drugmaker Merck & Co. announced Friday.
A five-day course of the drug, called molnupiravir, which is designed to introduce errors into the genetic code of the virus, was found to cut Covid hospitalizations and related deaths in half, according to a company press release. Merck said it intends to ask health officials in the United States and around the world for emergency authorization for the pill.
If it gets authorization, molnupiravir would be the first oral antiviral medication for COVID-19.
“This is going to change the dialogue around how to manage COVID-19,” Merck Chief Executive Robert Davis said.
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received molnupiravir within five days of Covid symptoms had about a 50 percent reduction in the rate of hospitalization and death as patients who received a placebo pill. The study tracked 775 adults with mild-to-moderate Covid who were considered at higher risk for severe disease due to health problems such as obesity, diabetes, or heart disease.
The results that were released by the company and have not been peer-reviewed. Merck said it plans to present them at a future medical meeting.