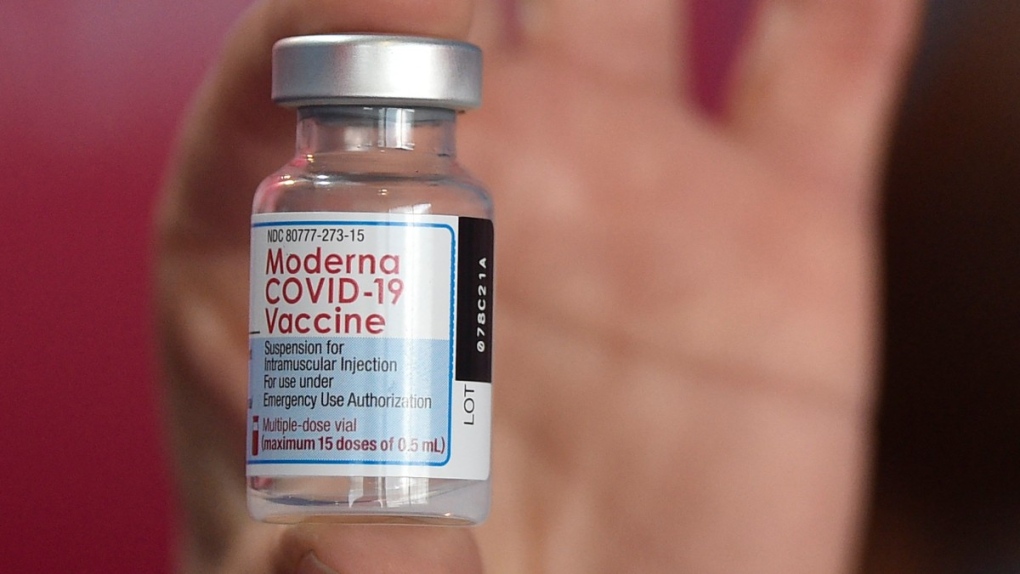
Swedish health authorities have suspended the use of Moderna’s COVID-19 vaccine for those ages 30 and under, saying the move was done out of precaution.
The reason for the pausing is “signals of an increased risk of side effects such as inflammation of the heart muscle or the pericardium” — the double-walled sac containing the heart and the roots of the main vessels, Sweden’s Public Health Agency said in a statement. “The risk of being affected is very small.”
Anders Tegnell, Sweden’s chief epidemiologist, said they “follow the situation closely and act quickly to ensure that vaccinations against COVID-19 are always as safe as possible and at the same time provide effective protection” against the disease.
Moderna’s vaccine was given the green light for use in anyone 18 and over across the 27-nation European Union in January. It has also been licensed in countries including Britain, Canada, and the US, but so far its use hasn’t been extended to children. To date, the Pfizer/BioNTech vaccine is the only one approved for children under 18 in Europe and North America.
The reason for the pausing is “signals of an increased risk of side effects such as inflammation of the heart muscle or the pericardium” — the double-walled sac containing the heart and the roots of the main vessels, Sweden’s Public Health Agency said in a statement. “The risk of being affected is very small.”
Anders Tegnell, Sweden’s chief epidemiologist, said they “follow the situation closely and act quickly to ensure that vaccinations against COVID-19 are always as safe as possible and at the same time provide effective protection” against the disease.
Moderna’s vaccine was given the green light for use in anyone 18 and over across the 27-nation European Union in January. It has also been licensed in countries including Britain, Canada, and the US, but so far its use hasn’t been extended to children. To date, the Pfizer/BioNTech vaccine is the only one approved for children under 18 in Europe and North America.