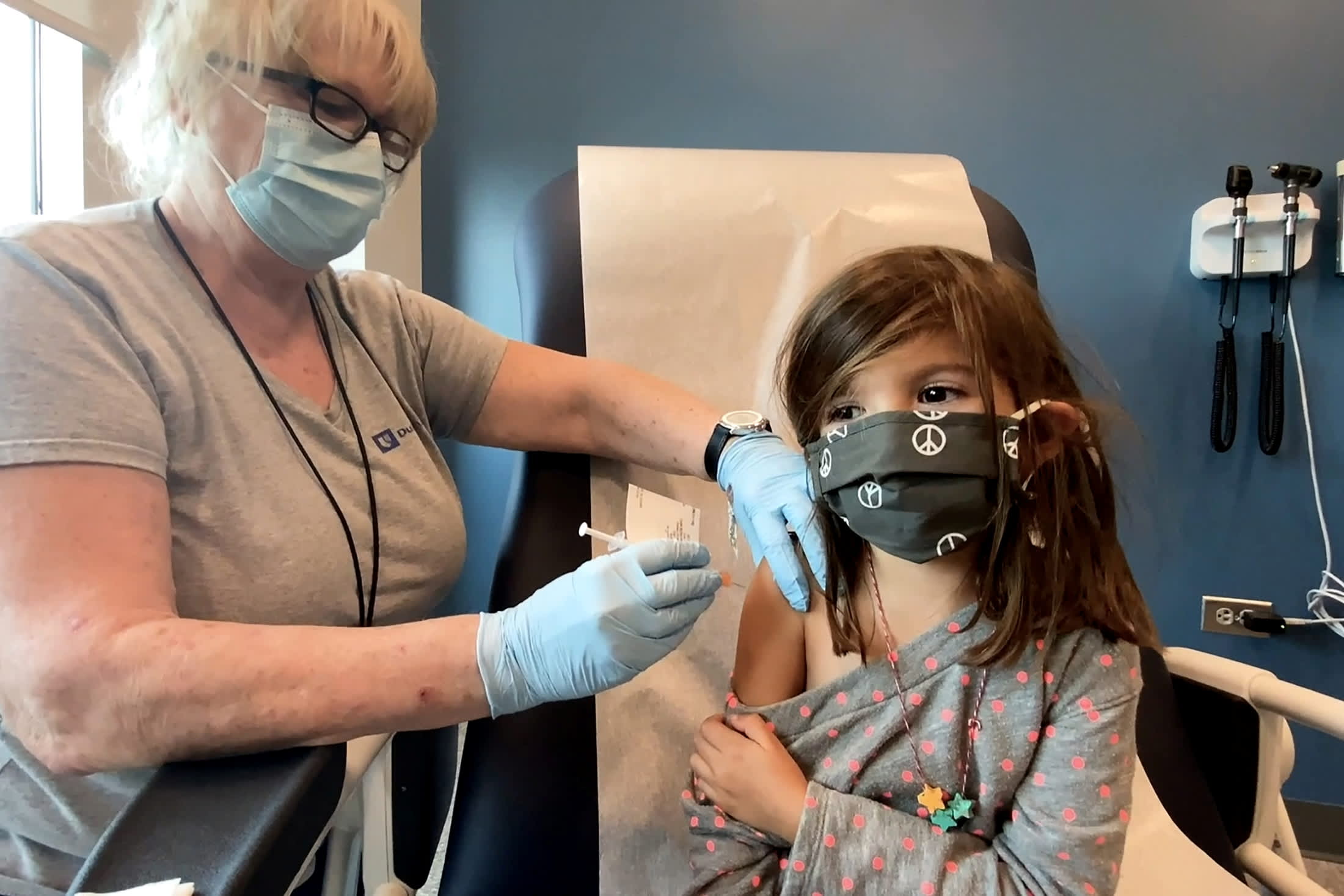
The US Food and Drug Administration on Friday authorized the Pfizer Inc. and BioNTech SE coronavirus vaccine for children aged 5 to 11 years, making it the first COVID-19 shot for young children in the United States.
The shot will not be immediately available to the age group. The US Centers for Disease Control and Prevention still needs to advise on how the shot should be administered, which will be decided after a group of outside advisers discuss the plan on Tuesday.
The FDA decision is expected to make the vaccine available to 28 million American children, many of whom are back in school for in-person learning.
Pfizer’s COVID-19 vaccine appears safe and nearly 91 percent effective at preventing symptomatic infections in 5- to 11-year-olds, according to a study. It also said the shots were well tolerated in young children, producing side effects comparable with those seen in a study of people ages 16 to 25.
Only a few other countries, including China, Cuba, and the United Arab Emirates, have so far cleared COVID-19 vaccines for children in this age group and younger.
The shot will not be immediately available to the age group. The US Centers for Disease Control and Prevention still needs to advise on how the shot should be administered, which will be decided after a group of outside advisers discuss the plan on Tuesday.
The FDA decision is expected to make the vaccine available to 28 million American children, many of whom are back in school for in-person learning.
Pfizer’s COVID-19 vaccine appears safe and nearly 91 percent effective at preventing symptomatic infections in 5- to 11-year-olds, according to a study. It also said the shots were well tolerated in young children, producing side effects comparable with those seen in a study of people ages 16 to 25.
Only a few other countries, including China, Cuba, and the United Arab Emirates, have so far cleared COVID-19 vaccines for children in this age group and younger.