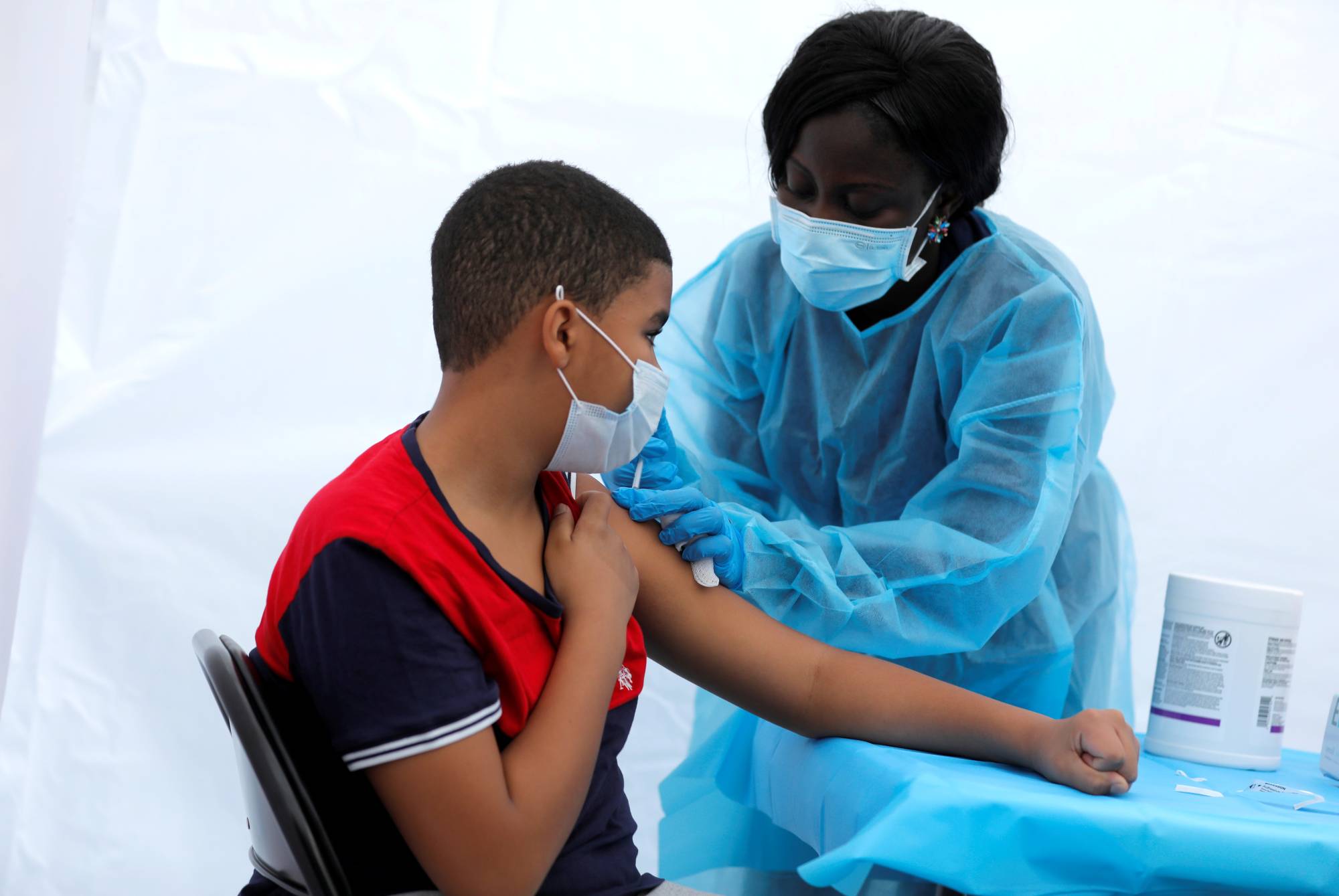
Pfizer and BioNTech said Tuesday they began submitting a formal request to US health regulators for emergency use of their Covid vaccine for children aged over six months and under five years.
If the Food and Drug Administration (FDA) authorizes the two-shot regimen, it will become the first Covid vaccine available to this age group in the United States.
In a tweet soon after the announcement, the FDA said it will hold a meeting on February 15 to consider the request.
“As hospitalizations of children under 5 due to COVID-19 have soared, our mutual goal with the FDA is to prepare for future variant surges and provide parents with an option to help protect their children from this virus,” said Pfizer CEO Albert Bourla in a statement.
Bourla said kids under 5 will ultimately need a third dose to have the best protection against omicron and future Covid variants. By getting the first two-doses FDA authorized, parents can start getting their kids vaccinated while they wait for the third dose, Bourla said.
“If two doses are authorized, parents will have the opportunity to begin a Covid-19 vaccination series for their children while awaiting potential authorization of a third dose,” he added.
To limit side effects for this young age group, Pfizer chose to significantly decrease the dosage of its vaccine, opting for only three micrograms per jab versus 30 for those over 12 years old, and 10 for ages five to 11.
The company’s researchers concluded last fall that low doses of the vaccine provided protection in children up to two years old but not in those aged two to five, and announced in December they would add a third dose to their trials.
Data on the three-dose regimen is “expected in the coming months and will be submitted to the FDA to support a potential expansion” of this initial request, Pfizer and BioNTech said in a press release.
If the Food and Drug Administration (FDA) authorizes the two-shot regimen, it will become the first Covid vaccine available to this age group in the United States.
In a tweet soon after the announcement, the FDA said it will hold a meeting on February 15 to consider the request.
“As hospitalizations of children under 5 due to COVID-19 have soared, our mutual goal with the FDA is to prepare for future variant surges and provide parents with an option to help protect their children from this virus,” said Pfizer CEO Albert Bourla in a statement.
Bourla said kids under 5 will ultimately need a third dose to have the best protection against omicron and future Covid variants. By getting the first two-doses FDA authorized, parents can start getting their kids vaccinated while they wait for the third dose, Bourla said.
“If two doses are authorized, parents will have the opportunity to begin a Covid-19 vaccination series for their children while awaiting potential authorization of a third dose,” he added.
To limit side effects for this young age group, Pfizer chose to significantly decrease the dosage of its vaccine, opting for only three micrograms per jab versus 30 for those over 12 years old, and 10 for ages five to 11.
The company’s researchers concluded last fall that low doses of the vaccine provided protection in children up to two years old but not in those aged two to five, and announced in December they would add a third dose to their trials.
Data on the three-dose regimen is “expected in the coming months and will be submitted to the FDA to support a potential expansion” of this initial request, Pfizer and BioNTech said in a press release.